What are the 4 Risk Categories of SaMDs?

There are many variables that go into identifying and classifying software as a medical device. It’s important to remember that SaMD is not software which supports the functions of medical hardware (that’s an SiMD).
To determine if software qualifies as SaMD, two questions must be asked:
- Does the software act as a medical device? This means there must be a medical benefit to its use as well as patient risk if it faulters or is incorrectly used.
- Can the software work independently from other devices? SaMDs can take data from other devices, but if the software supports a medical device, and that medical device does not work without that software, then it is considered SiMD, not SaMD.
In addition to these questions and other characterization rules / definition statements, SaMDs are also categorized by risk level. This means SaMDs are separated by the importance of the information provided and the healthcare situations and the conditions it is intended to be used in. Here are the 4 different categories.
How Software as a Medical Device is Categorized
SaMDs are categorized using numbers I through IV, where Category I has the lowest impact on patients or public health, and Category IV has the highest. This impact calculation is based on two main factors: How vital the information given by the SaMD is and the condition or time sensitivity in which the information is used. Let's take a closer look at each category and some potential examples.
Category IV SaMDS
Category IV SaMDs have the highest impact on patients and deal with life and death situations. The information or data given is time-critical. Examples include SaMDs that perform analysis to diagnose and make treatment decisions for situations like meningitis in children, acute stroke patients, cancer lesions, etc.
Category III SaMDs
Category III SaMDs also are involved with life and death situations, but unlike Category IV devices, the data or information is not time critical. Some examples include devices that make sound to alert apnea patients of interrupted breathing during sleep and devices that plan out and provide parameters when aiding in the treatment of a tumor.
Category II SaMDs
Category II SaMDs are involved in serious conditions, but not life threatening ones. A few examples include devices that analyze different blood tests to provide recommendations when diagnosing health issues pertaining to kidney function, cardiac risk, etc. and devices that provide visualization of a patient’s anatomical structure to help when placing a catheter, markers, etc.
Category I SaMDs
Category I SaMDs are used in the lowest threat level of health issues. Some examples include devices that collect medical data like ECG rate, heart rate, and walking speed of a rehabilitation patient or a device that uses data to proactively anticipate an occurrence of an asthma episode.
Categorization Factor 1: The Significance of the Information
This is the first factor that decides which category is right for a SaMD, and it can be broken down into 3 groups.
- Treat or diagnose
- Drive clinical management
- Inform clinical management
Categorization Factor 2: The State of the Situation or Condition
The second factor analyzed is how urgent or time sensitive the information given by the SaMD is. There are also 3 groups that this factor can be broken into.
- Critical
- Serious
- Non-serious
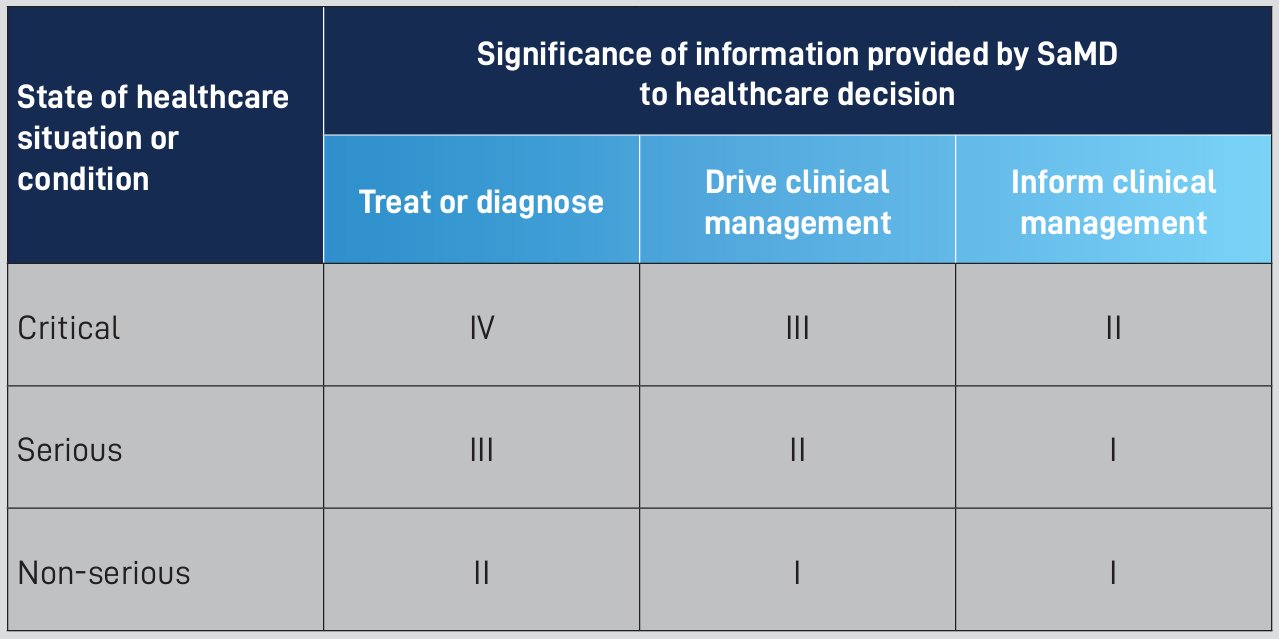
When the 2 factors are placed together in a table, you can see how each category is formed. For example, Critical information to treat or diagnose a patient is a category IV SaMD. If the SaMD is still used to treat and diagnose a patient, but for non-serious reasons, that SaMD would be put into category II. You can see that the risk level decreases as you move to the right and down, and increases as you move left and up.
If you are planning on or already developing a SaMD, it’s vital that the developers understand how the SaMD would be categorized. Each category has its own guidelines in order to be approved by the FDA or the EU.
Have additional questions about developing and designing SaMDs? We’d love to hear from you! Use the button below and we’ll be in touch soon.
